Perspective
is everything.
Across the pharma lifecycle, an accurate and complete market view is essential to stay ahead of the curve and uncover emerging healthcare trends.

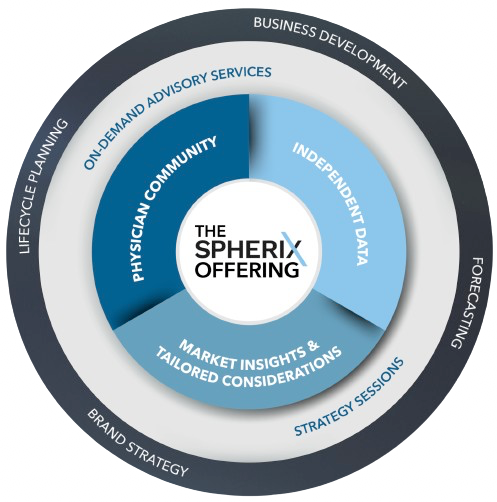

Gain an unbiased perspective of the market and its potential, independently assess launches, understand the patient journey and prescribers' perspectives, and gauge the impact of disruptive events.

Interactive on-site or virtual sessions with Spherix therapeutic experts, highlighting commercially relevant analyses, contextual data, and custom insights tailored to your business.

Fortify your strategy and tactics with our industry experts who are well-equipped to deliver strategic advisory services across the product lifecycle.
Gastroenterology Indications Covered:
Visit the Spherix portal for the full spectrum of our Gastroenterology offering
Download the Gastroenterology publication plan






04.08.2024
Pfizer’s Velsipity poised to outpace BMS’ Zeposia in ulcerative colitis market, but must head off further competition: analysts
04.08.2024
As Humira biosim sales languish, Boehringer Ingelheim plots layoffs in pivot to hybrid marketing model
04.05.2024
US Gastroenterologists Project Swift Uptake of Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) for the Treatment of…
03.22.2024
Gastroenterologists Slow to Adopt Eli Lilly’s Omvoh, Pfizer’s Velsipity, and Takeda’s subcutaneous formulation of Entyvio for…